FDA alerts patients of another recall of medicine used to treat high blood pressure
The US Food and Drug Administration declared they are recalling a medicine used to treat high blood pressure for a possible cancer risk due to contamination. This time, SciGen is also recalling certain lots of Irbesartan.
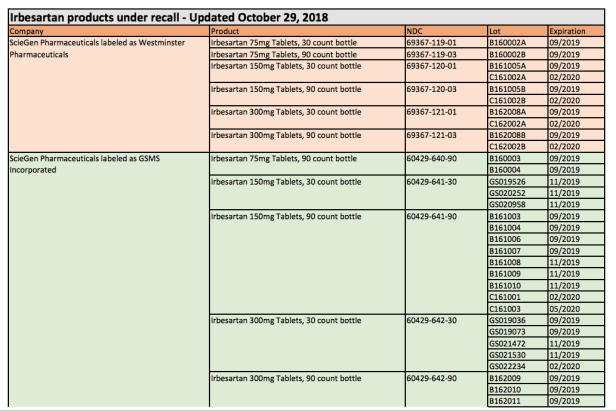
According to the FDA list, the recalled drugs will have “Westminster Pharmaceuticals” and “GSMS Inc.” on the label.
On July, the FDA recalled Valsartan, a drug used to treat high blood pressure and heart failure, due to contamination.
Irbesartan, an angiotensin II receptor blocker or ARB, blocks a potent chemical in the blood called angiotensin, causing muscle surrounding blood vessels to contract from binding with angiotensin II receptors.
Follow us on our Twitter account, @amomama_usa, to learn more.
At the point when the chemical binds, it limits the vessels, and that can cause high blood pressure.
Recently, the FDA stated that the recalled drugs might contain an N-Nitrosodiethylamine (NDEA), used in gasoline as a stabilizer and to make liquid rocket fuel.

Source: Freepik
This is the first non-valsartan drug product that the agency has discovered to contain the NDEA impurity.
Valsartan was contaminated with NDEA or NDMA, N-nitrosodimethylamine, an impurity that is also viewed as a conceivable cancer-causing agent by the US Environmental Protection Agency.
Source: Freepik
The FDA is testing all ARBs for these contaminations. It needed to plan a special test after it discovered that some ingredients imported from one company in China, Zhejiang Huahai Pharmaceuticals, were tainted.
The FDA put Zhejiang Huahai Pharmaceuticals on an import alert toward the end of September, which implies all dynamic pharmaceutical items and completed items made by the company won't be allowed to enter the United States.

Source: Freepik
As indicated by the FDA, not all medicine containing Valsartan or Irbesartan is recalled. This recall affects about 1% of the Irbesartan drug products in the US market.
The FDA said that if your drug is on the recall list, take it until the point when your doctor or pharmacist gives a replacement.
Source: coxrare.files.wordpress.com
The FDA also said it would keep on testing all products containing Valsartan and comparable medications for the presence of impurities.
The information in this article is not intended or implied to be a substitute for professional medical advice, diagnosis or treatment. All content, including text, and images contained on news.AmoMama.com, or available through news.AmoMama.com is for general information purposes only. news.AmoMama.com does not take responsibility for any action taken as a result of reading this article. Before undertaking any course of treatment please consult with your healthcare provider.
